top of page
mass
amount
molar mass
concentration
solution volume
gas volume
molar gas volume
Avogadro
constant, L
number of
entities, N
H3.1 WRITING AMOUNT–AMOUNT RATIOS MATHEMATICALLY - 2
The problems which follow deal with the key idea about amounts ratios and the interpretation of stoichiometric coefficients.
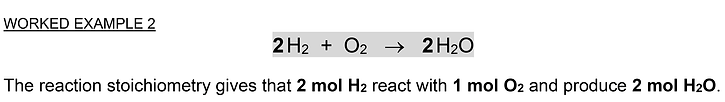
Often these are expressed mathematically as so-called de Donder relations

after the famous Belgian theoretical physicist (1873 – 1957).
The amounts ratio of interest and its most convenient representation often depend on how a problem is worded and what quantity needs to be calculated.

bottom of page



