mass
amount
molar mass
concentration
solution volume
gas volume
molar gas volume
Avogadro
constant, L
number of
entities, N
CALCULATING ON THE SIDE OF SUCCESS – Module S(2029): TITRIMETRIC ANALYSIS - THEORY & PRACTICE
S. TITRIMETRIC ANALYSIS: THEORY & PRACTICE
S1. BACKGROUND, FEATURES & PROCEDURES
S1.1 QUANTITATIVE ANALYSIS
Quantitative analysis is concerned with determining the amounts of known substances present in a sample of material. Several methods are available to the chemist including volumetric analysis, gravimetric analysis and a variety of advanced physical methods such as analytical electron microscopy. At this level, detailed knowledge is generally only required of titrimetric analysis - formerly called volumetric analysis. This is a powerful technique used in a variety of ways by chemists in many different fields.
S1.2 CHARACTERISTICS OF REACTIONS SUITABLE FOR TITRIMETRIC ANALYSIS
In titrimetric analysis the volumes of solutions of reacting substances are measured using burettes and pipettes.

Analysis is completed by determining the volume of a standard solution needed to react completely with the analyte. The point at which this condition is achieved is termed equivalence point or stoichiometric point.
We estimate the position of this equivalence point by observing some physical change in the solution; the point at which this physical change is observed is the end point.
A suitable indicator exhibits a colour change very close to the equivalence point.
To be suitable for an analysis of this type a reaction should:
1. be rapid - heating may be necessary, e.g., oxalate (ethanedioate) + permanganate
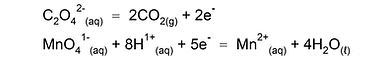
2. proceed reasonably far towards completion, i.e., high K – see later;
3. be described by a balanced chemical equation, i.e., NO side reaction occurring;
4. have an equivalence point detectable by a suitable end point.
Properties that have been used in determining end points include:
1. colour due to reagent, product or indicator;
2. turbidity due to the formation of an insoluble phase;
3. electrical conductivity of the solution (R) (conductametric titrations);
4. potential between a pair of electrodes (V) (potentiometric titrations);
5. refractive index;
6. electric current through solution (I) (ampèrometry).
In titrimetric analysis dilute solutions are used for a number of reasons, particularly expense of materials, the need to reduce the violence of reaction and to avoid changes in concentration - as occurs to many pure substances due to their hygroscopic nature or to their reacting with constituents of the air.
Titrimetric methods can be classified in four categories:
1. neutralization (acid-base) titrations;
2. redox titrations;
3. precipitation titrations, e.g.,
titrant
analyte
precipitate
end point
- red ppt.
4. complexometric titrations (using esp. EDTA).
S1.3 STANDARD SOLUTIONS
1.3.1 Preparation of Standard Solutions
If a reagent is available in the pure state, a solution of definite concentration is prepared simply by weighing out a mass equivalent to 1.000 mol, or a definite fraction or multiple thereof, dissolving it in the solvent, usually distilled water, and making up the solution to a known volume.
When the reagent is not available in the pure form as in the cases of most alkali hydroxides, some inorganic acids and various deliquescent substances, solutions of the approximate concentration required are first prepared. These are then standardised by titration against a solution of a pure substance of known concentration. It is generally best to standardise a solution by a reaction of the same type as that for which the solution is to be employed, and as nearly as possible under identical experimental conditions. The titration error and other errors are thus considerably reduced or are made to cancel out. This indirect method is employed for the preparation, among others, of solutions of most acids (for HCℓ, the constant-b.p. mixture of definite composition can be weighed out directly, if desired), NaOH, Ba(OH)2, KMnO4, and Na2S2O3.
1.3.2 Primary Standard Substances
For a substance to be classed as a primary standard it must meet the following criteria.

be available in a highly pure state
be stable in air
be able to undergo stringent analysis itself
it should have a high molar mass
be easily soluble in water
in solution, during analysis, it must undergo complete and rapid reaction
In practice, an ideal primary standard is difficult to obtain, and a compromise between the above ideal requirements is usually necessary. Note that substances of higher purity than primary standards are classified although they need not concern us here.
1.3.3 Sources of starting material for standard solutions
Solutions of known concentration can be prepared in several different ways depending on the nature of the analyte and/or the concentration required:
(a) weighing out a solid material of known purity, dissolving it in a suitable solvent and diluting to the required volume;
(b) weighing out a liquid of known purity, dissolving it in a suitable solvent and diluting to the required volume;
(c) diluting a standard solution previously prepared in the laboratory;
(d) diluting to 1.00 L from an ampoule a concentrated analytical solution obtained pre-packaged
from a commercial chemical supplier.
Some of the substances satisfying the criteria for primary standards, and therefore suitable for the preparation of standard solutions in the four inorganic reaction categories, include:

Titrimetric classification
Commonly employed primary standards
Acid-base reactions
Redox reactions
Precipitation reactions
Complexing reactions
sodium carbonate
constant b.p hydrochloric acid
potassium hydrogenphthalate
sulphamic acid
benzoic acid
potassium bromate
potassium iodate
iodine
sodium oxalate
potassium dichromate
arsenic(III) oxide
pure iron
silver
silver nitrate
sodium chloride
potassium chloride
Ag
potassium bromide (via the bromate)
NaCℓ
silver
silver nitrate
sodium chloride
various pure metals
Hydrated salts, generally, do not make good standards because of the difficulty of efficient drying. However, those salts which do not effloresce, such as sodium tetraborate decahydrate, Na2B4O7.10H2O, and copper(II) sulphate pentahydrate, CuSO4.5H2O, are found by experiment to be satisfactory secondary standards.
A secondary standard is a substance which may be used for standardisations, and whose content of the active substance has been found by comparison against a primary standard.
S1.4 TITRIMETRIC PROCEDURES, TECHNIQUES & SKILLS
1.4.1 Important distinction between accuracy and precision
In everyday language, these two terms are usually treated as synonyms, whereas in the context of scientific work they have quite distinct meanings.
The term 'precision' is used in two senses. The first is, properly, ‘sensitivity’; applied to a method of measurement, this indicates the smallest difference between two quantities that can be detected reliably, e.g., the sensitivity of top-pan balances is 0.001 g, so that one speaks of weighing to a precision of 1 mg. The second refers to the reproducibility of a repeated measurement, or a repeated operation concluding with a measurement, and is a measure of the scatter of the results.
Modern analysis makes a further distinction between the terms ‘reproducible’ and ‘repeatable’. Determinations made on the same day in rapid succession are classed as ‘repeatable’ analysis. However, if the determinations had been made on different days when laboratory conditions might have varied, these data would be considered ‘reproducible’. So, a within-run precision (repeatability) and a between-run precision (reproducibility) are distinguished.
The term ‘accuracy’ refers to the difference between the result of a measurement, or a process concluding with

a measurement, and the ‘true value’. Commonly, it is used for the difference between the true value and the mean of
a number of repeated measurements.
In contrast, precision has primarily to do with the number of significant figures to which a measurement can be made.
It reflects the technological quality of instrumentation and the extent to which error has been minimized by statistical means.
With titrimetric glassware, the most precise instrument is the pipette. With special care, a precision of 8 parts per million (ppm) can be obtained with a 25 mℓ pipette.
The limiting factor in volumetric titrimetry is set by the burette, in which readings between graduation marks have to be made by estimation.


The precision of titrimetric flasks is set by the diameter of the neck, and, with care, 3 parts in 10 000 can be attained, but normally
a precision of 0.02 % can be expected.
Zelda F. Scott, CC BY-SA 3.0
creativecommons.org/licenses/by-sa/3.0
via Wikimedia Commons
With a ring graduated 50 mℓ burette, the liquid can be set to a mark such as zero, to 1 part in 5000. The limit for a titration volume is therefore about 0.04 %.
The use of these instruments is outlined in general terms on pages 10-16 of the Flipbook S(2029).
Apparatus itemized in the table below measures with decreasing precision as the list is descended.

PRHaney, CC BY-SA 3.0
creativecommons.org/licenses/by-sa/3.0
via Wikimedia Commons
Recommended reading, certainly well before any significant practical work is undertaken, is available at
The sub-sections below are covered in Flipbook S(2029): TITRIMETRIC ANALYSIS - THEORY & PRACTICE
1.4.2 Weighing by difference & use of analytical balance
1.4.3 Use of the calibrated flask. Preparation of standard solutions
1.4.4 Use of the pipette. Precise transfer of liquids
1.4.5 Use of the burette. Precise volumetric titration
1.4.6 Performing a titration
1.4.7 Handling of calibrated glassware
1.4.8 Labelling and storing solutions



